Finding the right partner for private label cosmetics USA[^1] is exciting, but it is also where most new founders make fatal mistakes. Are you choosing a partner based solely on the lowest price per unit, ignoring the hidden compliance risks that could shut your business down?
The shift to mandatory FDA oversight means you can no longer rely on a handshake. If your manufacturer isn't compliant, your brand takes the fall. (Max 30 words).
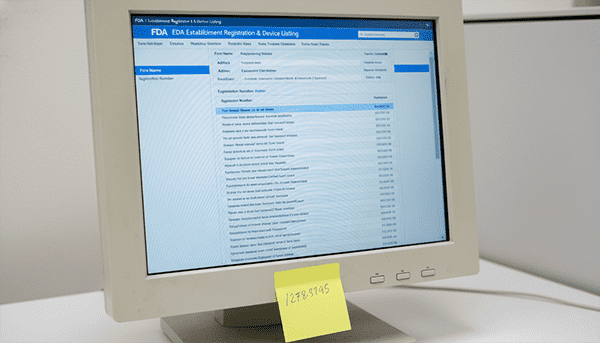
To verify a FDA registered cosmetic manufacturer[^2], you must request their 7-digit FEI (FDA Establishment Identifier) number. Do not accept a PDF certificate; verify the number directly on the FDA’s public database to ensure the facility status is "Active" and legally permitted to manufacture your goods.
A flashy website or a booth at a trade show doesn't guarantee a compliant facility. Let’s walk through the exact due diligence steps I use to vet suppliers, ensuring your brand is built on solid ground rather than a regulatory sinkhole.
Don't Let Compliance Gaps Destroy Your Brand: The Ultimate Due Diligence Checklist?
Blindly trusting a manufacturer’s sales rep is a recipe for disaster. If their facility isn't up to code, your brand pays the price in recalls, fines, and reputation damage.
Your due diligence must go beyond pricing. You need to validate their FDA registration status, confirm their US Agent contact, and audit their ISO 22716[^3] (GMP) certification. This "triangulation" confirms they are a legitimate cosmetic contract manufacturing[^4] partner.
When I first started in supply chain management, I saw brands accept "FDA Certificates" that looked official but were actually Photoshop creations made by third-party agencies. The FDA does not issue certificates. They issue data points.
To protect your investment, you must perform a "Compliance Audit[^5]" before you pay a single dollar in deposits. This is not just about following the law; it is about scalability. If you grow and retailers like Sephora or Ulta want to carry you, they will demand this paperwork. If your factory can't provide it, you lose the deal.
| Feature | The "Red Flag" Factory | The Compliant Partner (Camellia Labs) |
|---|---|---|
| Registration Proof | Sends a PDF "Certificate of Registration" from a consultancy. | Provides a 7-digit FEI Number[^6] for live verification. |
| GMP Standards[^7] | Claims "We follow GMP" but has no documentation. | Holds a valid, third-party audited ISO 22716[^3] certificate. |
| US Presence | No US Agent; difficult to contact during US hours. | Has a registered US Agent for FDA correspondence. |
| Transparency | Refuses to share the exact facility address. | Transparent about location and allows virtual/physical audits. |
A "cheap" factory that lacks these credentials will cost you more in the long run. When the FDA enforces MoCRA[^8], products from unregistered facilities will be detained. You will pay for storage, destruction fees, and legal counsel. Paying a slightly higher unit cost for a compliant manufacturer is your insurance policy.
Why Is the FEI Number[^6] the Only ID That Matters?
Many offshore factories try to confuse new founders with business licenses or export permits. None of these matter to the US government if the facility lacks an FEI.
The FEI (FDA Establishment Identifier) is the unique tracking number assigned to a facility by the FDA. It is the only valid proof that a FDA registered cosmetic manufacturer[^2] is recognized by the US government. Without it, you cannot list your products.

I cannot stress this enough: The FDA system is database-driven. When you eventually file your Product Listings (which is mandatory), the portal will ask for the manufacturer's FEI. If you type in a fake number, or if the factory hasn't renewed their registration, the system will reject your product.
How to Verify an FEI Number[^6]:
- Ask Direct Questions: Do not ask "Are you registered?" Ask "What is your FEI number?"
- Use the Portal: Go to the FDA's "Cosmetics Direct" or the generic establishment search portal.
- Check the Status: You are looking for the status "Active."
- Match the Address: Ensure the address in the database matches the address on your shipping invoices.
The "Middleman" Trap: Many companies posing as manufacturers are actually trading companies. They will refuse to give you the FEI because it reveals the actual factory they are outsourcing to. At Camellia Labs, we believe in transparency. As a brand owner, you are legally responsible for the supply chain, so you have a right to know exactly where your products are made. If a partner hides their FEI, they are hiding their identity.
Is Your "Safety Substantiation[^9]" File Actually Audit-Proof?
Under MoCRA[^8], assuming your product is safe because "no one has complained yet" is now illegal. You need scientific data backing every single SKU you sell.
A complete Safety Substantiation[^9] dossier must include quantitative formula data, Preservative Efficacy Testing[^10] (PET), stability reports, and a Toxicological Risk Assessment[^11] (TRA). Without these documents, your product is considered "adulterated" by the FDA, leading to immediate recall risks.

One of the most common questions I get is, "Sarah, do I really need to pay for all this testing?" The answer is yes. Private label cosmetics USA requirements have changed. The "Safety Substantiation[^9]" clause in MoCRA[^8] puts the burden of proof on you.
Here is the breakdown of what a compliant "Product Information File[^12]" (PIF) should look like. A good manufacturer will help you coordinate these tests.
- Preservative Efficacy Testing[^10] (PET / USP <51>):
- What it is: We inject bacteria and mold into the cream to ensure the preservative system kills them.
- Why you need it: To prove your product won't grow mold on the customer's shelf.
- Stability & Compatibility:
- What it is: 3 months of testing at high temperatures (45°C) and in the final packaging.
- Why you need it: To prove the packaging doesn't leak toxins into the formula and the formula doesn't separate.
- Toxicological Risk Assessment[^11] (TRA):
- What it is: A toxicologist reviews every ingredient's concentration against global safety limits.
- Why you need it: This is the gold standard for "Safety Substantiation[^9]."
While you pay for the third-party lab fees, your cosmetic contract manufacturing[^4] partner should provide the "Quantitative Formula" (exact percentages) directly to the safety assessor. At Camellia Labs, we facilitate this transfer under NDA, protecting our IP while ensuring you get the compliance documents you need.
Verifying your manufacturer isn't about being difficult; it's about being professional. Your brand's longevity depends on a supply chain that can withstand FDA scrutiny. At Camellia Labs, we don't just make products; we provide the FEI registration, safety data, and transparency you need to grow without fear. Let's build a brand that is safe, legal, and ready for the global stage.
[^1]: Explore this link to understand the essential steps for launching a successful private label cosmetics brand in the USA. [^2]: This resource will guide you on how to verify and select a compliant FDA registered manufacturer for your cosmetics. [^3]: This link provides insights into ISO 22716 certification and its importance for cosmetic manufacturers. [^4]: Discover the ins and outs of cosmetic contract manufacturing to make informed decisions for your brand. [^5]: Find out how to perform a compliance audit to protect your brand from regulatory issues. [^6]: Learn about the significance of the FEI Number in ensuring compliance for cosmetic manufacturers. [^7]: Learn about Good Manufacturing Practices (GMP) and their importance in cosmetic production. [^8]: Understand the implications of MoCRA on cosmetic regulations and compliance requirements. [^9]: Explore the concept of Safety Substantiation and its critical role in cosmetic product safety. [^10]: Learn about Preservative Efficacy Testing and its importance in ensuring product safety. [^11]: This resource explains the significance of Toxicological Risk Assessments for cosmetic safety. [^12]: Discover the essential components of a Product Information File to ensure compliance.